Abstract
Introduction: The clinical presentation of mantle cell lymphoma (MCL) is heterogeneous, ranging from indolent disease that does not require immediate treatment to rapidly progressive disease that responds poorly to aggressive immunochemotherapy. Several small retrospective studies suggested that active surveillance or delayed treatment is feasible in select patients with an indolent disease course. A shorter diagnosis-to-treatment interval (DTI) is a strong predictor for inferior survival in patients with aggressive non-Hodgkin lymphomas such as diffuse large B-cell lymphoma (DLBCL) and peripheral T-cell lymphoma (PTCL), which likely correlates with an aggressive disease biology and clinical presentation. However, the impact of DTI on survival has not been well studied in patients with MCL. In this study, we utilized the National Cancer Database to investigate the feasibility of active surveillance or delayed treatment in patients with MCL and to explore the potential impact of DTI on survival of MCL patients requiring treatment.
Methods: The National Cancer Database was used to identify adult patients (≥ 18 years) with MCL diagnosed from 2004 through 2017. Patients were divided into three groups based on their time to initial treatment: early treatment [ET] (treatment started within 3 months), delayed treatment [DT] (treatment started after 3 months) and surveillance [SUR] (watch and wait without treatment). Baseline clinical characteristics were compared between the 3 groups using Chi-square test. Survival analysis was performed using the Kaplan-Meier method and log-rank test. Statistical analysis was performed using SPSS version 25.
Results: A total of 27,867 patients with newly diagnosed MCL were included in this study, 995 patients (4%) in the SUR group, 21,425 patients (77%) in the ET group, and 1,052 patients (4%) in the DT group. There were no major differences in the baseline characteristics among the three groups including demographics (age, sex, ethnicity), socioeconomic factors (annual income, insurance status, place of residence), comorbidity scores, and Ann Arbor stage. Compared to patients in the SUR and DT groups, patients in the ET group were more likely to have B symptoms (ET=27% vs DT=16% vs SUR=8%, p<0.001) and were less likely to be managed at an academic center (ET=40% vs DT=53% vs SUR= 51%, p<0.001) (Table 1).
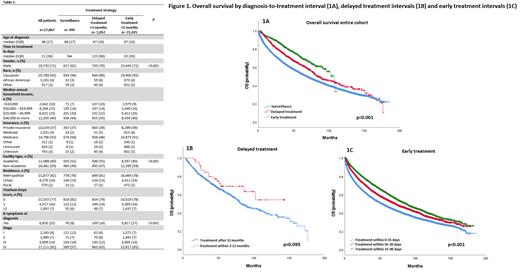
The median follow-up was 38.2 months for the entire cohort. There was a significant difference in OS among the three groups, with a median OS of 107 months (95% CI = 92.7-122.7) in the SUR group, 84.9 months (95% CI = 74.2-95.7) in the DT group, and 68.9 months (95% CI = 66.8-70.9) in the ET group (p=0.001) (Figure 1A). Among patients in the DT group, a shorter DTI was associated with a trend of inferior OS, with a median OS of 82 months (95% CI = 73.2-90.8) for those with a DTI of 3-12 months and not reached (95% CI not calculable) for those with a DTI > 12 months (p=0.095) (Figure 1B). Among the ET group, a shorter DTI was associated with shorter OS, with a median OS of 53 months (95% CI = 50.2-55.8) for patients with a DTI ≤ 15 days, 71.7 months (95% CI = 67.1-76.3) for those with a DTI of 16-30 days, and 87.1 months (95% CI= 82.8-91.4) for patients with a DTI of 31-90 days (p<0.001) (Figure 1C)
Conclusion: In this retrospective analysis, patients with newly diagnosed MCL who were managed with an active surveillance strategy or delayed therapy had a better survival compared to patients who underwent early therapy. These findings validate the feasibility of delayed therapy in select patients with MCL. In patients who undergo early therapy, a shorter DTI is associated with an inferior survival outcome, mirroring findings in DLBCL and PTCL. A shorter DTI is likely associated with more aggressive disease biology and clinical presentation.
Munoz: Pharmacyclics/Abbvie, Bayer, Gilead/Kite Pharma, Pfizer, Janssen, Juno/Celgene, BMS, Kyowa, Alexion, Beigene, Fosunkite, Innovent, Seattle Genetics, Debiopharm, Karyopharm, Genmab, ADC Therapeutics, Epizyme, Beigene, Servier: Consultancy; Gilead/Kite Pharma, Kyowa, Bayer, Pharmacyclics/Janssen, Seattle Genetics, Acrotech/Aurobindo, Beigene, Verastem, AstraZeneca, Celgene/BMS, Genentech/Roche.: Speakers Bureau; Millennium: Research Funding; Janssen: Research Funding; Seattle Genetics: Research Funding; Pharmacyclics: Research Funding; Genentech: Research Funding; Incyte: Research Funding; Portola: Research Funding; Merck: Research Funding; Celgene: Research Funding; Gilead/Kite Pharma: Research Funding; Bayer: Research Funding; Physicians' Education Resource: Honoraria; Seattle Genetics: Honoraria; Targeted Oncology: Honoraria; OncView: Honoraria; Kyowa: Honoraria. Paludo: Karyopharm: Research Funding. Habermann: Seagen: Other: Data Monitoring Committee; Incyte: Other: Scientific Advisory Board; Tess Therapeutics: Other: Data Monitoring Committee; Morphosys: Other: Scientific Advisory Board; Loxo Oncology: Other: Scientific Advisory Board; Eli Lilly & Co.,: Other: Scientific Advisor. Nowakowski: Nanostrings: Research Funding; Blueprint Medicines: Consultancy; TG Therapeutics: Consultancy; Kymera Therapeutics: Consultancy; Incyte: Consultancy; Zai Labolatory: Consultancy; Daiichi Sankyo: Consultancy; Bantham Pharmaceutical: Consultancy; Karyopharm Therapeutics: Consultancy; Curis: Consultancy; Selvita: Consultancy; Ryvu Therapeutics: Consultancy; Kyte Pharma: Consultancy; Genentech: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Celgene/Bristol Myers Squibb: Consultancy, Research Funding; MorphoSys: Consultancy. Wang: TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Eli Lilly: Membership on an entity's Board of Directors or advisory committees; MorphoSys: Research Funding; Genentech: Research Funding; Novartis: Research Funding; LOXO Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; InnoCare: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal